ooohhh boy this note is so messy, I will sort this out in a few days
Optical Methods
Contents
• Principles
and instrumentation of uv and visible spectroscopy, and its application to
qualitative and quantitative analysis.
• Spectrofluorimetry
- primary and secondary events, quenching, sensitivity and discrimination,
instrumentation.
• An outline of the principles and
applications of atomic absorption spectrophotometry and emission flame
spectrophotometry
The Electromagnetic Spectrum
Energy of Electromagnetic Radiation
Energy E = hν where h = Plank’s constant
ν = frequency
Frequency ν =
speed (c) / wavelength (l)
Frequency ν a 1 /
wavelength (l)
Energy a 1 / wavelength
ie. shorter wavelength = higher energy
Absorption 1
• Electrons
exist in precisely defined energy levels
• An
atom absorbs light of a given wavelength
• Uses
energy to move an electron to higher energy level
– An
outer shell electron
– Ground state → Excited state
• Light
of a particular energy is
absorbed for a particular change in energy level
• Gives
an absorption spectrum
• Light
of a particular energy is
absorbed for a particular change in energy level
• Gives
an absorption spectrum
• Energy
difference between electron states is in region of uv & visible light
• Atoms
absorb light in uv-visible range
• We
see reflected (non absorbed light)
• GS:
Ground state
• E1:
1st Excited state
• E2:
2nd Excited state
• E3:
3rd Excited state
Colour
• Due
to Energy levels
• The
internal energy of molecules changes in steps
• Energy,
E = hn
(h = Plank’s constant, n = frequency of light, n = 1 / l , l
= wavelength of light)
• A
result of electrons changing energy levels
Atomic &
Molecular Absorption Spectra
• Atomic
spectra
– Transitions
between states
– Eg
s0 → s1, s0 → s2
– Only
light of ‘right’ wavelength is absorbed
– →
Exact energy for a transition (electron jump)
– Line
spectra are obtained
• Molecular
spectra
– Molecules
absorb light of several different wavelengths
– Electrons
in different atoms
– Vibrations
of bonds absorb energy
– Mixture
of signals causes a broadening from a line to a band or peaks around a
wavelength
Molecular Absorption
Spectrum
• Molecule
absorbs light
• Electrons
from several atoms move to several different energy levels and bonds vibrate
• Molecule
absorbs light of several different wavelengths
• Absorption
spectrum:
• Spectrum
can be a characteristic molecular signature
• Measured
in a spectrophotometer
What colour is chlorophyll?
Does the spectrum show what you would expect?
History of Spectrophotometry – Colorimeter
• In
1853 a device was invented that shows which light in the spectrum was absorbed
• Colorimeters
were then used to detect red of
haemoglobin or the green of chlorophyll
• Technique
was used to conclusively identify blood at a crime scene
UV- Visible Spectrophotometry
• A
spectrophotometer detects the amount of radiant light energy absorbed by
molecules
• Shine
light from near infra red through to ultra violet spectrum through a sample
• Detect
which wavelengths are absorbed
Spectrophotometer
• 6
basic components:
– a
light source
– a
prism or diffraction grating
– an
aperture or slit
– a
sample cell (of known path length)
– a
detector (a photocell or photoelectric tube)
– and a digital meter to display the output of
the phototube
• Dual
beam or single beam
Basics of a spectrophotometer
Principle 1
• Incident
light is reflected from a diffraction grating.
• It
is split into its component colours or wavelengths, which then diverge.
• Sections
of the projected spectrum can be either blocked or allowed to pass through the
slit so that only one wavelength will pass to the other sections of the
spectrophotometer.
• The
position of the grating is adjustable so that the region of the spectrum
projected on the slit can be changed.
Principle 2
• Light
passes through the slit to the sample tube
• Light
not absorbed by the sample tube travels to the phototube
• Light
creates an electric current µ
to the number of photons striking the phototube.
• If
a digital meter is attached to
the phototube, the electric current output can be measured and recorded.
• The
scale is usually calibrated in two ways:
– percent transmittance: 0 to 100 and
– absorbance, or optical density units 0 to
2.
Preparation
• Choose
the wavelength:
– The
diffraction grating is adjusted so that the desired wavelength of light passes
through the slit
– Usually
the wavelength of light that is most absorbed by the compound (e.g. 515nm for
glucose)
– Alternatively
a scan will give the full spectrum
• Zero-ing
using a blank:
– The
output of the phototube must be adjusted or calibrated to correct for drift in
the electronic circuits or contaminants between the source and the detector
– Dual
beam instruments allow simultaneous zeroing or baseline correction
Specs
• Single
beam instruments
Applications
• Identifying
compounds: All solutions of chemical compounds absorb light of specific
wavelengths.
• Determining
concentrations: the amount of light absorbed is µ
to the conc. of a compound
To analyze water quality, measure glucose
levels, monitor bacterial growth, perform protein assays and many applications
in the medical, forensic & environmental sciences, physics, and
biotechnology.
Proteins
Trp & Tyr have an absorbance peak at 280nm used in
protein quantification (Beer Lambert law A= ecl)
Beer Lambert law
• Relationship
between absorbance, at a given wavelength and concentration
• A= ecl
Where: A = absorbance
e =
molar extinction coefficient (M-1 cm-1or
dm3.mol-1cm-
1)
l = length of the light path (usually 1 cm)
c = concentration of the solute (M)
Learn this formula you will use it a lot
Use of Beer Lambert
law
• A= ecl
A solution of NADPH has an absorbance at 340nm of A = 0.622
what is the concentration?
e(NADPH)
= 6220 M-1 cm-1
l = 1 cm
c = concentration of the solute (M)
A= ecl > c = A/el
c = 0.622/6220 x 1
c = 0.0001 M (or
0.1mM)
Always remember the
units
Molecular Absorption
Spectrum
• Spectrum
can be a characteristic molecular signature
• Measured
in a spectrophotometer
•
Molecule absorbs light of different wavelengths
•
Electrons from several atoms/ bonds move to
several different energy levels and bonds vibrate
Applications
• Identifying
compounds: All solutions of chemical compounds absorb light of specific
wavelengths.
• Determining
concentrations: the amount of light absorbed is µ
to the conc. of a compound
To analyze water quality, measure glucose
levels, monitor bacterial growth, perform protein assays and many applications
in the medical, forensic & environmental sciences, physics, and
biotechnology.
UV spectrophotometer
(Double beam)
(Double beam)
Instruments Measuring in Ultra Violet
2 Light Sources:
• Tungsten
bulb delivers visible light > 320 nm
• Deuterium
lamp delivers uv 160 – 375 nm
• Automatic
switch over at 360 nm
Optics:
• Must
be silica-Glass absorbs uv light.
Light Detection:
• Two
photocells required.
• ‘Blue’
photocell measures 200 – 500 nm
• ‘Red’
photocell measures 350 – 800 nm
Sample preparation for UV
Solution or
suspension (cloudy) – consider solubility and appropriate solvents
Choose appropriate cuvette (sample holder)
Cuvettes may be:
- Optically transparent plastic – ok for aqueous samples, not for organic. (480-600 nm) inexpensive and disposable, easily scratched
- Optical grade Glass – ok for organics (400-900 nm), more scratch-resistant
- Fused silica or quartz – must be used for wavelength ranges (190-750 nm), very expensive –Care!
Cuvettes
Dual Beam Recording Spectrophotometers
• Response
of the photocells changes with wavelength, cuvettes & buffers may absorb
light
• → spectrophotometer must be re-zeroed against
a blank for every wavelength
• Problem:
What happens with a ‘scan’ across wavelengths
• Double
beam allows blank to be continually reset
• Double
beam instrument: the light beam is split into two parts
• →
One part is passed through a blank solvent cell → Other part passes through the
sample cell
• Absorbtion
spectra can thus be obtained in a continuous scan
• Alternative:
newer single beam specs record, save and subtract a baseline scan
Transmittance
When light intensity entering the sample (blank) = I0
and light intensity leaving the sample = I
I / I0
= transmittance
Transmittance depends on:
Concentration of the absorbing molecule (c)
Distance travelled by light through the absorbing substance
-
light path (l)
A constant, characteristic of the absorbing substance (e)
Absorbance
Transmittance, I / I0 = 10-ecl
log I /
I0 = -ecl
ie. - log I / Io
= ecl
-log I / I0 (ie. –log transmittance) is called absorbance
(A) a unitless quantity
ie. A = ecl
This is the origin and derivation of the Beer – Lambert
laws.
Beer-Lambert Law
states that the absorbance of a sample (A), at a particular
wavelength, is proportional to the concentration (c) of the sample (M or mol dm-3)
and the path length of the light (l) through the sample (cm)
A = e c l
also expressed as A = -log10 (I/Io) =log(Io/I)
e is the molar extinction coefficient and is a measure of how strongly the compound absorbs at that wavelength
(units of M-1cm-1 or dm3.mol-1cm-1)
A = e c l
also expressed as A = -log10 (I/Io) =log(Io/I)
e is the molar extinction coefficient and is a measure of how strongly the compound absorbs at that wavelength
(units of M-1cm-1 or dm3.mol-1cm-1)
Beer-Lambert Law A = e
c l
A linear relationship between A & c
Molar extinction coefficient e is the slope of line
l =1 cm
(think of y = mx + c : A = ec + 0)
Molar extinction coefficient e is the slope of line
l =1 cm
(think of y = mx + c : A = ec + 0)
Important Practical Point
Remember that absorbance A = -log transmittance.
When A = 1,
transmittance = 0.1 ie. 10%
When A = 2,
transmittance = 0.01 ie. 1%
When A = 3,
transmittance = 0.001 ie. 0.1%
Many digital instruments will give A = of 2.5 or 3. NB. Here
A is much less than 1% of the incident light → very inaccurate
Don’t use absorbances higher than about 1.5
Ideal range:
0.1<A<1
Quantification by UV-Vis
• Beer
Lambert Law: A = e c l
– Last
lecture, but need to know e
• Comparison
of unknown to standard
concentrations
– Plot
a standard curve
• Derivatisation
& Colorimetric reactions
– Non
absorbing substance is reacted with reagent which converts it/is converted to a
derivative which absorbs light
– Requires
a standard curve
Practical points about colorimetric assays
• Standards
treated exactly the same way as
the test sample/s
• If
a test sample gives an absorbance higher than the highest value of the
calibration curve it should not be
diluted to make it fit the curve.
– there
might not be enough derivatisation reagent present to react all the sample if a
sample of higher concentration than the highest standard is used
– Further
dilution might alter ‘blank’
– Results
in a false low measurement.
Examples of substances measured by colorimetric reactions
Many modern Biomedical tests are colorimetric assays:
• Trinder’s
assay detects glucose by oxidation of a colourless dye → A515 ↑ (pink)
• Reducing
sugars eg. Glucose and fructose, derivatised using dinitro salicylic acid
(DNSA). The derivative absorbs light maximally at 540nm.
• Protein
reacted with Folin reagent forms a derivative which absorbs light maximally at
750nm.
Non derivatised
protein absorbs light at 280nm, but the Folin assay allows measurement in the
visible range, and is more sensitive.
• Other
protein assays include: Bradford, Biuret, BCA & Lowry – all have a colour
change proportional to [Protein]
• Amino
acids form a derivative with ninhydrin which absorbs maximally at 570nm.
• Phosphate
forms a derivative with ammonium molybdate which absorbs light maximally at
600nm.
Clinical Chemistry Analyser
• Roche
Cobas 8000
• up
to 8,400 tests per hour with a total of 270 reagents
• Multiple
analytes from 1 patient sample
• Provides
a complete clinical chemistry profile
• Based
on spectrophotometer
BCA Assay
• Determination
of unknown protein concentration
• Colorimetric
assay
• Use
standard curve
• Protein
causes a blue/violet colouration
• Measure
at l
= 562 nm
Standard Curve
• Use
protein standards: dilutions of a known 1mg/ml BSA solution to plot a standard
curve
Calculate the concentrations of the unknowns from standard
curve using the absorbances measured
Enzyme assays in a spectrophotometer
S + E ES P + E
1) Production
of the product
2) Consumption
of substrate
e.g. NADH or other chromophore
Commonly by determination of Absorbance values using a
spectrophotometer
A real example: Glucose-6
Phosphate Dehydrogenase
Follow production of
the NADPH molecule
Absorbance = ε.c.l
(ε = molar extinction coefficient, c = concentration, 1 =
1cm)
Concentration = Absorbance/ ε
Concentration/min = Absorbance/ min. ε
Vo = slope/ ε
Slope: 0.00348abs/sec
For Glucose-6 Phosphate Dehydrogenase assays, ε = 11.5 dm3.mol
-1.cm -1 Vo
= 0.00348abs/sec/11.5 dm3.mol -1.cm -1
Vo = 0.000302608
mol/sec
Glucose-6 Phosphate Dehydrogenase and Glucose-6 Phosphate Dehydrogenase
with Aurintricarboxylic Acid Ammonium salt (ATA) an enzyme inhibitor
Turbidimetry and Nephelometry
• Spectrophotometers
can be used to measure light scatter from particles.
• Can
be used to estimate the amount of particulate material eg bacteria in growth
medium.
• The
method is known as turbidimetry.
• A
purpose built instrument called a nephelometer
can be used for exactly the same purpose.
A simple turbidimetry assay for aggregation
Conclusion
• UV-Vis
is a key tool in many areas of bioscience study and diagnostics
• Quantitative
data can be obtained from the use of:
– Beer-Lambert
Law A = e c l
– Colorimetric
assays
• For
more info and worked examples see following slides – a similar calculation to
the NAD+/NADH calculation will be in the exam
Special uses of spectrophotometers
For example it is
possible to measure substances with
similar but different absorbtion spectra when mixed in the
same sample.
An important example is the co-factor nicotinamide adenine
dinucleotide (NAD+) and its reduced form, NADH.
These two substances have identical absorbtion spectra
below 300nm, but NADH has an absorbance peak at
340nm, where NAD+ does not absorb.
By measuring at 340nm the concentration of NADH can be
measured in the presence of NAD+.
By measuring at
260nm and 340nm the concentrations of
NAD+ and NADH in a mixture can be measured, as in
the
example below.
Protein (absorbance
max 280nm) and nucleic acid
(absorbance max 260nm) can be measured in a mixture by
a similar method. This is not quite so easy since protein
has some absorbance at 260 and nucleic acid at 280nm,
so an algorithm ( a formula specifically for this calculation)
has to be used in this case.
NAD+ and NADH measurement in a mixture
Molar absorbance of NAD+ at 260nm = 18,000
Molar absorbance of NADH at 260nm = 18,000
Molar absorbance of NAD+ at 340nm = 0
Molar absorbance of NADH at 340nm = 6220
A mixture of NAD+ and NADH measured in a cell of
1cm
light path gave the following absorbances:
A at 260nm = 1.4
A at 340 nm = 0.25
Using the Beer – Lambert law
A = Ecl (or c = A / El)
[NAD+] + [NADH] = 1.4 / 18,000 x 1 M = 7.7 x 10-5M
[NADH] = 0.25 / 6220 x 1 M
= 4.0 x 10-5M
Therefore [NAD+] = 7.7 x 10-5 – 4.0 x 10-5M
= 3.7 x 10-5 M
Linked assays
Because NADH can be measured in the presence of NAD,
enzyme reactions which use NAD / NADH as co-factors
(dehydrogenases / reductases) can be measured by
following production of NADH from NAD at 340nm.
The method of enzyme measurement is so simple and so
useful that it is used to measure enzymes which do not use
NAD / NADH as co-factors by linking them to enzymes
which do. This type of enzyme assay is called a linked
assay.
Measurement of metabolite concentration using NAD / NADH
The dehydrogenase
assay and linked assay methods can
also be used to measure metabolite concentrations in
unpurified mixed samples, such as plasma, or tissue
homogenates.
The methods can only measure concentrations within quite
narrow limits, but most samples can be diluted into the
measurement range.
The methods are specific because they use enzyme active
sites to identify the material to be measured. The sample
can therefore be a constituent of an impure mixture.
Electrons, Energy
& Light – Revision
n Electrons
(e-) exist in defined energy levels
n Light
has energy E = hn
(h = Plank’s constant, n = frequency of light)
n Atoms
& bonds absorb light of given wavelengths
n Use
energy to move an e- to higher energy level
n Energy
is also lost from high energy e-
Fluorescence 1
- Light absorbed
- → e- jumps from ground state to a vibrational energy level of excited state
- But e- falls to base level of the excited state within 10-12 seconds
- e- then falls back to the ground state within 10-8 seconds
- Absorbed energy is released from molecule
- This may be:
- kinetic energy (heat)
- emitted as a quantum of light (less common)
→ Re-emitted light is fluorescence
Re-emitted light is fluorescence.
About 10% of molecules which absorb light also fluoresce
→ Fluorescence
spectroscopy / fluorimetry /
spectrofluorimetry
Fluorescent Biomolecules
• Drugs
– Aspirin,
morphine,barbiturates, tetracyclins
• Vitamins
– Vitamins
A, B6 & E, riboflavins, nicotinamide
• Polutants
– Napthalene,
anthracene, benzopyrene
• Proteins
– Due
to tryptophans in the polypeptide chain
Characteristics of fluorescence 1
Stokes Shift
Stokes Shift
• Not
all the light energy absorbed is re-emitted
• Some
lost from the loss of vibrational energy
• Therefore:
– emitted
light has less energy than that absorbed
– ie.
it is emitted at a longer wavelength
• Difference
between wavelengths of maximum absorbance and of maximum emission is the Stokes shift
• Stokes shift = λmaxF
- λmaxA
Characteristics of fluorescence 2 Quantum Efficiency
• Not
all absorbed light is re-emitted as fluorescence
• →
Some energy is always lost as heat
• Less
light is emitted as was absorbed
• Ratio
of no. of photons absorbed to no. of photons fluoresced = quantum efficiency
• Stokes shift and quantum efficiency are
characteristics of a fluorescent molecule.
Measurement of Fluorescence 1
• Photons
fluoresced a conc. of fluorescent material
• Relationship
between fluorescent intensity and concentration is linear (not log., like
absorbance)
• Fluorescence
can be used to measure concentration
• Fluorescence
can be used over a wide concentration range
• →
very versatile technique when a molecule fluoresces
• Performed
in a fluorimeter
• Fluorescent
light is emitted from molecules → can be measured against a black background
• More
efficient to measure a small quantity of light against a black background than
to measure a small quantity of light absorbed from a bright beam
• Thus:
measurement of fluorescence more sensitive than absorbance.
• eg.
about 100mg of adrenalin can be measured by absorbance methods, but about 100
pg can be measured by fluorescence.
Absorbance v Fluorescence
Fluorimeter (or fluorometer)
• Measures
fluorescence
• Has
two monochromators (filters), to select the wavelengths of absorbed light
(excitation light), and emitted light (fluorescence)
• Fluorescent
light is emitted in all directions equally
• Fluorimeters
measure fluorescent light against a black background at right angles to the
excitation beam
• Detector
is a photomultiplier tube.
Thioflavin T (TfT) fluoresces when bound to aggregated
proteins
Here its used to measure Amyloidb
Advantages of Fluorescence Measurement
• Measurement
of fluorescence is more sensitive than absorbance measurement
• →
usually about 1000x more sensitive for same compound
• Sensitivity
is good enough to measure concs. of material released from cells and tissue
• Linear
relationship between conc. and fluorescence → method used over a wide conc.
Range
• Many
cyclic organic molecules with conjugated double bonds fluoresce inc. important
biological molecules eg…
– tryptophan
(in proteins)
– nucleic
acid bases (in NAD and ATP as well as DNA and RNA).
Disadvantages of Fluorescent Measurement
• Not
all molecules which absorb light fluoresce
• Fluorescence
varies with pH and temperature, therefore assays must be buffered and
temperature controlled
• Fluorescence
is easily affected by quenching
ie. absorbance of light by chemicals or coloured compounds present in samples
as contaminants.
Fluorescent Labels
• Fluorescent
molecules can be attached to other molecules by chemical linkers to act as
labels
• Fluorescent
labelled antibodies can be used to pinpoint specific antigens in cells can be
used to view living cells.
• Specific
cells can be labelled with fluorescent antibodies and collected using a fluorescence activated cell sorter (FACS).
• This
allows detection, measurement and collection of very specific cell types, and
is very important in diagnosis eg of leukaemias, and immunological research.
• DNA
is now sequenced using fluorescence labelled bases
• Proteins
can be engineered with fluorescent reporters eg green fluorescent protein
Green Fluorescent Protein
GFP = A jelly fish protein
Acts as a reporter when GFP DNA is fused to cDNA in
transfections
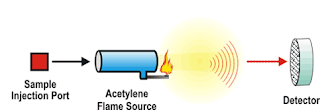
Atomic Spectroscopy
• Atomic absorption spectrophotometry
(flame absorption spectrophotometry)
• Atoms
dispersed in flame
• Measures
light absorbed at specific wavelengths by atoms eg Cu, Zn, Pb
• Relies
on Beer Lambert law
• Flame atomic emission spectrophotometry
(flame photometry)
• Measures
light from metal atoms in a flame
• Commonly
K+ ,Na+ & Ca+
in biological fluids
Atomic absorption
spectrophotometry
Flame atomic
emission spectrophotometry
• For
more info on atomic spectroscopy go to: http://www.resonancepub.com/atomicspec.htm











































I am glad that I saw this post. It is informative blog for us and we need this type of blog thanks for share this blog, Keep posting such instructional blogs and I am looking forward for your future posts.zeiss เลนส์
ReplyDeleteGreat blog! Thank you for sharing.
ReplyDeleteDownload Indian Doctors Network where you can network and communicate with executive committee members, senior doctors and mentors.