At my university
1st Year:
- Human Physiology (by Silverthorn)
- Biology (focus on cell, molecular, biochemistry and genetics - Sadava textbook sufficient)
- Chemistry and Statistics: introductory
- Biochemistry and Genetics (Stryer or Voet and Anthony Griffiths)
- Microbiology (Brock Biology Microbiology 13th)
- Human in Health and Disease (by Barbara J. Cohen)
2nd Year:
- Human Physiology and Pathology (detail on respiratory, cardiovascular, blood, endocrine, kidneys, histo)
- Laboratory Skills and Techniques (centrifugation, chromatography, optical methods, electrophoresis, immunoassays, etc.)
- Molecular Biology (by Craig, Molecular Biology: genome function or Lizabeth Alison)
- Metabolism (Essential Physiological Biochemistry by Stephen R. and Stryer/Voet)
- Molecular Genetics
- Medical Microbiology and Immunology
3rd Year:
- Medical/Clinical Biochemistry
- Immunology
- Infectious Disease Processes (more in depth of medical microbiology in level 5)
- Cellular Pathology and Hematology
- Clinical Immunology and Transfusion Science
- Independent Research Project
Others being taught in other institutions
- Histopathology
- Evidenced Based Medicine
- Nutrition
- Pharmacology
- Endocrinology
- Neuroscience/Neurobiology
- Cancer Biology
- Developmental Biology and/or Embryology/ Developmental Genetics
- Anatomy
- Histology
- Enzymology
- Proteins
- Chromosomes and Genes
- Genomics
- Proteomics
- Metabolomics
- Epidemiology
- Toxicology
- Virology, Parasitology, Mycology, etc.
- Biology of Ageing
- Biochemistry and Genetics of Disease
Resources: the most important in my opinion are Anatomy & Physiology, Voet/Stryer Biochemistry, Genetics, Microbiology, Molecular and Cell biology, Pathology, Immunology, The fundamental book series from biomedical sciences books may also be good for someone to get a grid on Biomedical Science!
Thursday, 27 December 2012
Monday, 24 December 2012
Centrifugation
Centrifugation – the basics
A centrifuge produces a centrifugal force many times greater
than the earth’s gravity, by spinning
the sample about a central axis.
Centrifugation is the
process of separating mixtures suspended in a liquid by applying centrifugal force.
A very slow centrifuge….
A rather faster centrifuge…
Particles of different size, shape, or density will sediment at different rates, depending
on the speed of rotation and distance from the central axis.
Centrifugal field G, operating on a particle P, spinning at
a distance r from the central axis of a centrifuge.
Centrifugal force
Is centrifugal force real?
For our purposes:
Centrifugal force acts in an outward direction when a
particle or object moves in a curved or circular path.
Exploded view of a centrifuge
A centrifuge:
a piece of equipment that rapidly spins a number of tubes
which contain a suspension of particles in a liquid
A typical centrifuge
The force which causes particles to move down in a
centrifuge tube:
Applied Centrifugal Field
Applied Centrifugal Field
• G = ω2 x r [ω
is the Greek omega]
• where
G is the applied centrifugal field (in cm per second squared: units of
acceleration)
• ω is the angular velocity of the rotor (in radians per second)
• r is the radial distance of the particle from the axis of rotation (in
centimetres)
Applied Centrifugal
Field and revolutions per minute
(revs.)
• The
angular velocity ω is more easily understood in terms
of revolutions per minute:
• ω = 2π
x (revolutions per
minute) / 60
• Therefore
since G = ω2 x r, the applied centrifugal field becomes:
• G = 4π2 x r x (revolutions per minute)2 / 3600
Equation to apply
for G in cm/s2
A pilot
in a fast aircraft executing a sharp turn or climb will experience a ‘g force’
several times that of gravity.
We can
also express Applied Centrifugal Force in a centrifuge as a ‘g force’
Relative Centrifugal
Field (RCF)
• The
centrifugal field is more commonly expressed in multiples of the gravitational
field of the earth (981 cm per second squared)
• Known
as the Relative Centrifugal Field (RCF)
and the units are in g:
• RCF = 4π2 x r x (revolutions per
minute)2
/
(3600 x 981)
• This
simplifies to
Relative Centrifugal Field (RCF) =
1.118 x r x
(revolutions per minute)2 x
10 -5
[ where the radius r is in centimetres (cm)]
1g = 981cm/s2
Equation to apply for RCF units of g
Applied and Relative Centrifugal Field
• The Applied Centrifugal Field G G= 4π2 x r x (revolutions per minute)2 / 3600
[in units of cm per second squared]
• and the Relative Centrifugal Field (RCF)
RCF=
1.118 x r x (revolutions per
minute)2 x 10 -5 [in
units of multiples of gravity]
[NOTE: G = RCF x g]
[the radius r is in centimetres (cm)]
Relative Centrifugal
Field (RCF)
QUESTION:
• A
fixed angle centrifuge spins at 20 000 revolutions per minute. The distance from the centre of the liquid
suspension in the centrifuge tube to the axis of rotation is 35 mm. What is the Relative Centrifugal Field
experienced by the particle?
• RCF= 1.118 x r x (revolutions per minute)2 x
10 -5
Example: calculation
of RCF
• Spins
at 20 000 revolutions per minute.
Distance from the centre of the liquid to axis of rotation is 35 mm (remember
to convert this to centimetres = 3.5 cm).
RCF = 1.118 x r x (revolutions per minute)2 x
10 -5
• RCF = 1.118 x 3.5 x (20000)2 x 10 -5
• = 1.118
x 3.5 x (4 x 108) x 10 -5
• = 1.118 x 3.5 x 4 x 103
• RCF = 15652 g [where g means
times the force of gravity]
Nomogram
Columns are (left to right): Radius (cm); RCF (g); Speed
(rev/min)
The
line gives an RCF of 100,000 or 1000g for a centrifuge radius of 10 cm and
speeds of 30,000 and 3,000 rev/min respectively.
Types of centrifuge
• Low-speed
centrifuges Swing-out rotors are
used in low-speed centrifuges (3000-6000 r.p.m.) for harvesting cells and
larger organelles (e.g. nuclei).
• Microcentrifuges Fixed-angle
rotors are used (up to 12 000 r.p.m) for higher-speed operation, for cells and
precipitates.
• Ultracentrifuges (speeds
greater than 30 000 r.p.m.) are used for very small particles e.g. biological
macromolecules.
Swing-out low-speed centrifuge and sample tube
Swing-out
centrifuges are used at speeds of 4000-10000 revs. per minute, with RCFs of
3000 – 7000g.
Mainly
used to collect material that sediments rapidly
eg PEI
resin from HCl
Table-top microcentrifuge
One of the most commonly used centrifuge types. Can reach
speeds of 12000-15000 rpm (RCF about 12000g).
Mainly
used to harvest small volumes of cells, or isolate microgram quantities of (for
example) proteins and nucleic acids.
Microcentrifuge has a fixed-angle rotor and uses
microcentrifuge tubes
Eppendorf’ tube
capacity 1.5ml
Smaller (0.5 ml or 0.2 ml) tubes require use of different
rotors
Floor-standing large centrifuge
For
centrifuging large volumes of a mixture at one time.
Centrifuge for 96-well plates
An ultracentrifuge
Ø Refrigeration
Ø Operates
under vacuum
Ultracentrifuges
• Refrigeration
is needed to counteract the heat generated during centrifugation at high speed,
to keep clinical and biological specimens stable.
• Evacuation
also reduces air friction.
• Analytical
centrifugation uses an ultracentrifuge with an optical system to observe the
settling of the particles.
Different types of centrifuges
Rotor types
Centrifuge with swing-out rotor
When
the rotor is in motion and the tubes are horizontal, why doesn’t the liquid
drain away?
Angled rotor
This is
the type most often used with microcentrifuge tubes. Another version is used
with very high speed centrifuges
Solid
construction; heavy; sample tubes have tops fixed in place during spinning.
Fixed angle rotors
• Are
ideal for pelleting during differential centrifugation to separate biological
particles with different sedimentation rates.
• The
pellet is the name given to the material which collects at the bottom of the
centrifuge tube. The liquid left above the pellet is the supernatant.
Vertical rotor
Ø Vertical
rotors use sealed centrifuge tubes
(diagram is not accurate!). Why?
Ø Samples
sediment across the diameter of the tube – short run times.
Centrifuge: application at UEL
Sample preparation for HPLC high performance liquid
chromatography:
example
Analysis of herbal
medicine tincture solutions and dried plants for active ingredients
HPLC column. Can be blocked by small particles. Remedy:
microcentrifuge the sample solutions before analysis and use the clear solution
above the pellet for analysis.
Centrifugal force
Centrifugal force acts in an outward direction when a
particle or object moves in a curved or circular path.
A centrifuge is a piece of
equipment that rapidly spins
a number of tubes which contain
a suspension of particles in a liquid
Sedimentation
Sedimentation
is the settling of solid particles through a liquid under the influence of a
gravitational or centrifugal field.
The speed at which a particle will settle in a liquid is
related to the size, shape and density of the particle.
The size of the particle is the major
determining factor in the settling (or sedimentation) rate of a particle, but
its density and weight also make a difference to the speed.
Sedimentation of different-sized particles
Factors controlling the sedimentation of a particle
• The
denser a particle, the faster it will sediment
• The
heavier the particle, the faster it will sediment
• The
denser the solution in which it is suspended, the slower a particle will
sediment
• If
the particle and solution are of equal density the particle will not sediment
• The
greater the centrifugal force, the faster it will sediment
Sedimentation and particle size
Particles
of different sizes can be separated by the difference in their sedimentation
rate.
Centrifugation
is a convenient method of increasing the speed of sedimentation of all the
particles in a mixture.
• But
the relative rates of sedimentation of different size particles are not
affected by the speed of the centrifuge.
• For
example if particles of size A sediment ten times faster than those of size B
at 1000 revs per minute (centrifuge speed), A also sediments 10 times faster
than B at 10,000rpm.
Rate of
sedimentation
• The
rate of sedimentation of a
particle in a solution (medium) is given by Stoke’s Law:
V= 2 x R 2 (ρp - ρm)
x g x RCF
9 η
• V
= sedimentation rate in cm per second;
• R is the radius of the particle in cm;
• ρp and ρm are the densities of
the particle and medium (solution) in gm/cm3
• g is the gravitational field in cm/sec2,
which should be multiplied by the Relative Centrifugal Field (RCF)
• η is the viscosity of the medium
(units are gm/sec.cm).
• V= 2 x
R 2 (ρp - ρm) x g x RCF
• 9 η
• where V = sedimentation rate in cm per second
• QUESTION: what if ρp and ρm are equal?
• - the densities of the particle and the medium
(the solution).
calculation of rate
of sedimentation
V= 2 x R 2 (ρp - ρm)
x g x RCF
9 η
• For
a particle of radius R
0.02 cm (diameter 400
microns) [1cm=10mm=10,000microns]
• particle
density ρp of 1.2
gm/cm3 in a liquid of density ρm 1.0 gm/cm3
• liquid
viscosity η of 1.00
• g=
980 gm/cm2 in a centrifugal field with RCF=1000g:
What is the Sedimentation rate (v) in cm/sec?
V= 2 x R 2 (ρp - ρm)
x g [1cm=10mm=10,000microns]
9 η
• Particle
radius R 0.02 cm (diameter 400 microns)
• particle
density ρp of 1.2
gm/cm3 in a liquid of density ρm 1.0 gm/cm3
• liquid
viscosity η of 1.00
• g=
980 gm/cm2 in a centrifugal field with RCF=1000:
Sedimentation
rate (v) is calculated as
• =
2 x (0.02)2 x (1.2 – 1.0) x 980 x 1000 /( 9 x 1)
• =
2 x 4 x 10-4 x 0.2 x 980 x 1000 / 9
• =
17.42 cm/sec
We may wish to calculate not the rate of sedimentation (in
cms per second), but the time taken for a particle to settle – this is more
useful to know.
Distances needed to calculate time for a particle to settle :
rt (radial distance to top of liquid)
rb (distance to bottom of tube)
Settling out time for a particle
t = 9 x η x (ln
rb/rt) x 3600
8 π2 (ρp - ρm) (rev/min)2
x R2
η is the
viscosity of the medium (units are gm, sec and cm).
rb and
rt are
the radial distance to bottom and top of liquid
ρp and ρm are the densities of
the particle and medium (solution) in gm/cm3
R is the
radius of the particle in cm;
Centrifugation
is used for two main applications: Preparative
and Analytical
Preparative is the separation of
components of cells etc into their components
Analytical produces information
on the quantities of components present.
Preparative also divides
into two types: 1) Differential
(this week) and
2)
Density gradient (next week).
Preparative centrifugation
1. Differential
Centrifugation
• uses
differences in sedimentation rate
• selectively
sediment out particles with particular properties
• applied
centrifugal field is increased step-wise
• at
each stage different types/sizes of particles can be collected
Differential centrifugation
The
longer a solution is centrifuged, the more the particles separate by size.
In
Differential centrifugation, the centrifuge is run for long enough for all the
particles of a certain size to pellet out, leaving the smaller sizes suspended
in the supernatant.
Differential centrifugation: separates into different
particle sizes
• Largest
particles separate out first (into the pellet)
• A
suspension of particles is centrifuged just long enough to pellet the largest
types.
• The
supernatant liquid is poured off into another centrifuge tube.
• But
some small particles also ended up in the pellet. What to do about it?
Sedimentation – getting clear separation into particle sizes
• Particles
of small size ‘trapped’ in the pellet.
• Answer:
remix the pellet with liquid to get another suspension; re-centrifuge until all
the large particles are in the pellet.
• After
this second stage, an even smaller number of small particles are ‘trapped’.
• The
supernatant liquid is added to the first supernatant liquid for centrifuging
out the next (smaller) size particles.
A useful rule for differential separation:
It has
been shown in practice:
to
achieve an effective separation between particles of two different sizes, they
must differ by an order of magnitude
ie –
one must have a diameter 10 times that of the other.
Angled rotor
Sedimentation patterns in different
centrifuge
rotors
centrifuge
rotors
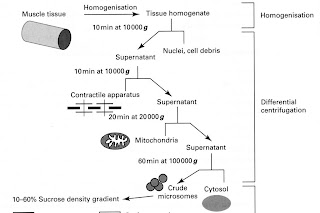
Practical example of Differential centrifugation
Differences
in sedimentation rate are used to selectively sediment out particles with
particular properties: example - fractionation of skeletal muscle homogenate. (see K.Wilson and J.Walker, Principles and Techniques of Practical
Biochemistry)
•
applied centrifugal field is increased step-wise
•
at each stage different types/sizes of particles
can be collected.
Example of a step-wise separation of a skeletal muscle
homogenate
Differential centrifugation Stage 1
Differential centrifugation Stage 1
• centrifuge
the mixture of 10% (w/v) of the
homogenate at 1000g (RCF) for 10 mins: pellet = cell nuclei plus debris
Differential centrifugation Stage 2
supernatant
from stage 1 is then re-centrifuged at 10,000g for 10 min; pellet = contractile apparatus.
Stage 3
supernatant from 2 re-centrifuged
at 20000g for 20 min; pellet = mitochondria
Stage 4
• supernatant
re-centrifuged at 100 000g for 60 min; pellet = crude microsomes; supernatant contains cytosol. [ultracentrifuge required]
• Ribosome from E.Coli.
• [Microsomes
are rich in ribosomes]
Centrifugation
is used for two main applications
Preparative
and Analytical.
Preparative
is the separation of components of cells etc into their components.
Analytical
produces information on the quantities of components present.
Preparative
centrifugation
also divides into:
also divides into:
1:Differential
The
centrifugation field is increased in stages.
2:Density gradient
- Rate zonal (particle size separation)
- Isopycnic (particle density separation)
1: Differential centrifugation
Differential
Centrifugation uses differences in sedimentation
rate to selectively sediment out
particles of different sizes.
BUT: Differential
Centrifugation is not very good at separating particles which are only slightly
different in size.
2:Density gradient
centrifugation
divides into:
divides into:
- Rate zonal (particle size separation)
- Isopycnic (particle density separation)
What is density gradient centrifugation?
In Density
Gradient centrifugation, the density of
the solution in the centrifuge tube increases from the top to the bottom
of the solution.
2: a. Density
Gradient
RATE ZONAL centrifugation
RATE ZONAL centrifugation
• Particles
are separated by size
• Different
sizes of particles move at different speeds down through the density gradient.
- RATE ZONAL centrifugation is like a race: the particles travel at different speeds towards the bottom of the centrifuge tube. Their size controls their speed.
- Particles which differ is size by 10% or more can be separated.
RATE ZONAL
centrifugation:
how it works
how it works
- layer a mixed sample in solution onto the top of a shallow pre-formed density gradient
- centrifuge
- larger particles will move faster through the gradient than the smaller
- distinct zones (bands) of different size particles
- Centrifugation is stopped before any band reaches the bottom of the tube.
2: Density Gradient
RATE ZONAL (particle size separation) – THEORY
If two
types of particle are very similar in density, but of different mass (that is,
radius, or size) the two types will sediment at different rates.
Sedimentation rate (Stoke’s Law) depends on
the radius squared (R is the radius):
V= 2 x R 2 (ρp - ρm)
x g x RCF
9 η
Rate-Zonal Density Centrifugation
• The
density gradient does not change very much down the tube
• Antibody classes all have very similar
densities, but different masses.
Separation based on mass
will separate the different classes, but separation based on density will not be able to resolve
these antibody classes
Criteria for
successful rate-zonal centrifugation
• Density
of the sample particle must be greater than that of the highest density portion
of the gradient
• The
pathlength must be enough for separation to occur
• Time
is important. Too long a run, and all
particles may pellet at the bottom of the tube.
Rate-zonal centrifugation
2: b. Density
Gradient ISOPYCNIC centrifugation
If a
particle and solution are of equal density the particle will not sediment.
Samples
are added to solutions in which the liquid density increases towards the bottom
of the centrifuge tube (and the solutions are then centrifuged).
Isopycnic
density gradient centrifugation
• Isopycnic
(from the Greek: isos: equal, pyknos: dense)
• Requires
a steep density gradient (the density increases fairly rapidly down the
centrifuge solution)
• During
centrifugation, once a particle reaches a part of the solution with the same
density as itself, it will not sink further.
Equilibrium Isopycnic density gradient centrifugation
Rate Zonal vs
Isopycnic density gradient centrifugation
2: Applications of density gradient centrifugation
• where
there is more difficulty in separating out a mixture – for example where there
are several kinds of large molecules or particles which must be separated from
one another.
• to
obtain fractions enriched in individual proteins from the supernatant obtained
from a low-speed centrifugation of broken cells.
• for
separation of protein molecules, high rotor speeds (often up to 70000 rpm) are
required.
Solutions used in forming a density gradient
Sucrose - a sugar
Glycerol
Ficoll – a polysaccharide
Percoll – a colloidal silica
Caesium Chloride – an alkali metal salt Disadvantage: may
damage biological structures
Density gradient types
for either Rate-Zonal or Isopycnic
for either Rate-Zonal or Isopycnic
Methods of forming density gradients
With a special mixing instrument
(will form linear, concave and convex gradients).
Or
A self-forming gradient (a
solution which forms a gradient just by centrifuging it).
Percoll will spontaneously form a LINEAR gradient when a
solution is centrifuged
Percoll solutions
contain colloidal silica particles coated with a thin layer of plastic. Centrifuging a solution will spontaneously
form a LINEAR gradient –known as a self-forming gradient. The Percoll colloid
settles to the bottom of the tube, making the solution denser from the top to
the bottom
Laboratory practical
is on isopycnic density gradient
- A gradient has been pre-formed in a centrifuge tube using Percoll
- Carefully pipette/layer the density marker beads (coloured) on top of the Percoll solution
Laboratory practical: isopycnic density gradient
- Centrifuge the solution. The beads will settle in coloured layers. Each colour represents one density.
- Measure the distance that each coloured layer has moved from the top of the solution.
Rf = (distance
travelled by a layer of beads/total distance)
distance travelled
by a layer
total distance
Analytical
Centrifugation
Analytical produces
information on the quantities of components present.
It usually requires the use of
an ultracentrifuge – high speeds are required.
A spectrometer is used to
monitor changes in the optical absorbance of the solution as the particles
sediment.
Uses of analytical centrifugation
• Determination
of the purity of macromolecules
• Determination
of the Relative Molecular Mass of molecules in solution
• Changes
in Relative Molecular Mass of molecules
• Study
of the binding sites (receptors) on proteins for cell signalling
Analytical rotor and absorption cell
Analytical ultracentrifugation
measuring changes in optical characteristics.
The rate of movement of a concentration boundary gives information on the biomolecule.
Subscribe to:
Comments (Atom)